Essays #1: Dancing in E. Coli
How molecules move through an E. Coli cell, a closer look at DNA storage, and the beauty of learning the details
There is a unique joy that comes from learning a topic in more detail. You’ll have a rough idea of what something is, and then a revelation — a paragraph or chapter or video or conversation — will make you realize that there’s so much more to it than meets the eye. It feels, almost, like a gentler, prolonged ‘eureka’ moment. Your mind perks up, excited to explore this new avenue and discover something you probably haven’t considered before.
I felt this way when I saw the structure and learned about the functioning of E.Coli in David Goodsell’s book, The Machinery of Life. Exploring, in detail, how a simple bacterium functions not only corrected some assumptions I had about cellular structures, but made me realize I had subconsciously held them in the first place, and, occasionally, never considered the specifics at all.
Take, for example, molecules moving inside a cell. I know that a cell disposes off waste; that messenger RNA goes to a ribosome to get translated into proteins; that enzymes bind to molecules to enable or disable reactions. But how, exactly, do these molecules move — how do they get where they need to go? I hadn’t really considered their inter-cellular journey. In my mind, I imagined they picked the fastest route to their destination, much like I would walking to a new coffee shop using Google Maps. But cells are a crowded place. They’re stuffed with molecules large and small: proteins, nucleic acids, amino acids, sugars, ATP, and other small molecules, all surrounded by water. Moving inside a cell is less like walking along a street, and more like bumping your way across a crowded nightclub floor. If you’re an enzyme looking for your target molecule, it’s more akin to hoping you’ll bump into it somewhere in the crowd, rather than getting to exactly where it is.
Finding out that the molecular interactions that sustain life happen by random chance, rather than intentional direction, felt a little troubling at first. I mean, chance? But again, the details reveal why this might be beneficial. Molecular interactions occur at specific orientations: the atoms or molecules connect best at some specific region, like two adjacent puzzle pieces. The crowded environment means two elements will spend more time next to each other, shuffling and bumping, which increases the likelihood of reactions. Crowding, and the barrage of bumping, also favours the assembling of molecules into larger complexes. It also means that molecular targeting (by enzymes, say) needs to be very specific — “a perfect match of shape and chemistry” — lest they react with some other molecule before running into the right one. Additionally, research by Penn State has shown that molecules move faster in crowded environments as long as the crowding is not uniformly distributed. That is, when there’s a region that’s more crowded than another nearby region, molecules are pushed towards the less crowded region faster than if the whole space was uniformly crowded or uniformly sparse. Chance, it seems, has more tricks up its sleeve than one might imagine.
Molecular transportation is a complex topic, and this method — diffusion — is just one of several ingenious ways molecules move around inside cells. There’s more to diffusion itself too, but another beautiful part of learning the details is that details tend to resemble Russian nesting dolls — there’s usually another layer to discover. This next layer, I’ll perhaps leave for a another essay.
How to (Not?) Store DNA
Another aspect of E. Coli that taught me something new is how DNA is organized inside the cell. The double helix shape of DNA is one of the most well-known biological shapes in the world. Ask a stranger what DNA stands for, and you might get a puzzled look; ask a stranger to draw DNA, and you will likely get some variation of two lines coiling around each other. I first learned about DNA in school, and its key aspects have been reinforced through nearly every biology-related article or book since. I know it consists of two complementary base pairs, adenine (A) and thymine (T), and guanine (G) and cytosine (C). I know it has a sugar-phosphate backbone. I know that it’s long — really long (over 4 million base pairs in E. Coli; approximately 3 billion in humans). And I know that DNA is primarily stored in the nucleus of cells (or in the nucleoid for prokaryotic cells like bacteria).
What I had not considered, however, was how it was stored. The nucleoid in E. Coli is a tightly-packed place, and its twisting and turning DNA, in order to fit in a space one hundredth its size, is squished in. The squishing results in something that I found as startling as I did astonishing: DNA, the most important molecule in this bacterium’s quest to reproduce, is not organized neatly, as one would imagine, but kept in a tangled, knotted mess. Looking at Goodsell’s beautiful watercolour below, it’s difficult to picture how any of the processes that involve DNA — reading the encoded information, translating a segment into RNA, splitting the strands for replication — happen at all.
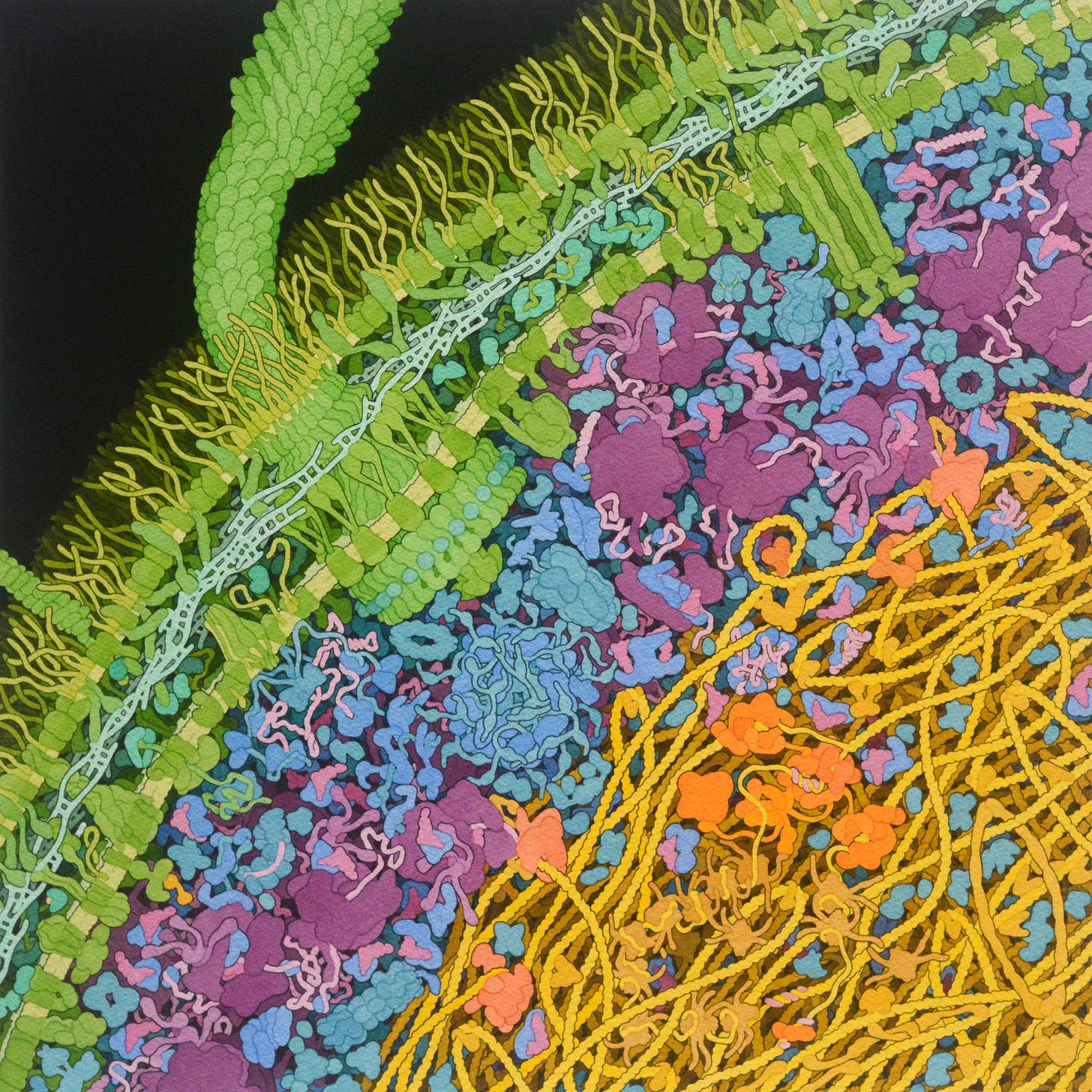
But, again, life has developed some clever, if not mildly concerning, tricks. There is an attempt at organization: to facilitate the squishing, a number of curious-looking proteins are employed. There’s SMC, resembling a twisted safety-pin, that holds together loops of DNA, and HU and Fis, that act as clips to ensure two sections stay together. The result isn’t going to impress cable management enthusiasts, but it’s seemingly functional.
Even more shocking than this wacky attempt at organizing is how E. Coli resolves tangles and knots to enable those vital DNA processes. An enzyme, DNA topoisomerase, untangles DNA segments by cutting it, “allowing strands to pass by each other”, and then reconnects them after. Pause, for a second, to consider how ridiculous this is: cutting and resewing as a solution to molecular tangles. (If only such a device existed in the macro-world — would’ve been really handy when my wired headphones got tangled in my pockets all those years ago).
In the off chance that it makes an error, the cell also has an audit and repair squadron — enzymes and proteins that search and fix DNA damage — operating continuously. One of the simpler ones, the MutM protein “searches for damaged guanine bases and removes them before they can lead to a mutation.” If the damage is more extensive, the RecABC system can “repair breaks in DNA by matching the broken DNA with an intact strand”, like a quality assurance analyst, or an editor performing a spell-check. (Which leads to a question: How does it know that the “intact” one is correct?)
So far, I’ve just written briefly about two phenomena in a segment of a single E. Coli cell. A lot of cellular trickery is preserved along species, but the living world is a diverse place, and a lot isn’t. Imagine what you might learn looking closely at rose petal cells, human heart cells, falcon eye cells, jellyfish cells. How many of these stupefying, astonishing — miraculous — cellular tricks are happening in my cells right now? I wonder: do they all also cut and sew back their tangled DNA? And do their molecules also move by bumping — dancing — across their crowded inner world? Perhaps these nesting dolls are for another day.

