The experiment that turned back biological time
The story, science, and effort behind the discovery of the induced pluripotent stem cell
While most articles about science focus on the impacts of breakthroughs or ground-breaking experiments, this essay aims to offer something different. My goal is to provide a genuine glimpse into the scientific process through the eyes of the scientists themselves. If you're not a researcher or haven't spent much time in a lab, consider this a window into what truly happens behind the scenes. And if you are a researcher or familiar with this field, please consider this as a tribute to your dedication — an homage to the countless hours, challenges, and perseverance that make scientific discovery possible.
One day in the early 1980s, a teenager from Higashiosaka, a bustling industrial city east of Osaka proper, accompanied his father to the hospital. Shozaburo, a middle-aged engineer, ran a family-owned factory with the help of his wife, designing and manufacturing components for sawing machines, the same factory his father ran before him. That day, he had a small accident – nothing critical, but it did require a blood transfusion.
Everything seemed fine for a while, but then one day he suddenly fell ill. The symptoms suggested hepatitis, but tests for hepatitis-A and hepatitis-B came back negative. This mystery illness slowly caused his condition to worsen; soon, a liver cirrhosis crept in. Suspecting his final days were approaching, he told his son that he should not take over the family business like he did, and he should become a doctor instead.
The son, then in his early twenties, looked up to his father immensely, and decided to follow his wishes and become a doctor. Astonishingly, his father lived to see him receive his M.D. from Kobe University, and even received medical treatments — injections and IV drips – from him while he was a resident at the Osaka National Hospital. But, sadly, a year later, in 1988, his father passed away, a fateful moment that would change the way a 26-year-old Shinya Yamanaka saw medicine, and his role in it.
Shinya would choose to specialize in orthopedic surgery, but would soon realize that he was not cut out for it. His hands lacked the dexterity to properly conduct surgery, and he routinely struggled to complete operations in a timely manner. His supervisors discouraged him from continuing on this path.
Feeling powerless after being unable to help his father despite his medical degree, Shinya too felt like this path wasn’t for him. A year after his father’s death, the virus that caused it was identified in the United States: hepatitis C. He realized, then, that you cannot treat what you don’t know, and found himself getting pulled in a new direction: medical research.
Trading the scalpel for the pipette, Shinya’s path led to a pivotal postdoctoral fellowship at the Gladstone Institute in San Francisco. There, he mastered the techniques of gene editing and embryonic stem cell culture, sparking a curiosity that would define his future research: how do these remarkable cells maintain their undifferentiated state?
After returning to Japan, Shinya Yamanaka struggled to find the funding and resources to continue the research he started. He nearly gave up and returned to medicine, but the breakthrough discovery of isolating human embryonic stem cells reignited his passion, illuminating the potential for curing patients using their own cells. When he got the opportunity to start at the Nara Institute, he decided to work on understanding these stem cells. While most labs explored how stem cells become specialized, Yamanaka posed a far more audacious question: can a differentiated cell return to its stem-cell state? Can you go the other way — can you turn back biological time?
The pluripotency puzzle
At the heart of this emerging field lay a cell of almost mythical potential: the embryonic stem cell. Sourced from the inner cell mass of a nascent embryo just days after fertilization, they are nature's primordial building blocks, the ultimate biological blank slate. They possess an extraordinary, almost magical, property called pluripotency — the potential to differentiate, to transform, into virtually any specialized cell type the body requires, from rhythmic heart muscle cells and complex neurons to insulin-producing beta cells or protective skin. Understanding, and perhaps replicating, this remarkable adaptability became a major goal of modern biology.
Yamanaka's audacious “reversing biological time” question didn't arise in a vacuum. The scientific world had already been buzzing with possibilities and grappling with limitations surrounding ES cells. The birth of Dolly the sheep in 1996, cloned from an adult udder cell, was a landmark moment. It dramatically proved that the genetic instruction manual in a specialized adult cell wasn't permanently fixed; it could theoretically be reset to generate a whole new organism. This fueled excitement about the potential of human ES cells. However, ES cell research, particularly in the United States, became deeply mired in ethical controversy because deriving these cells typically involved the destruction of human embryos. This led to significant restrictions on federal funding and spurred many scientists, including Yamanaka, to seek alternative ways to harness the power of pluripotency without relying on embryos.
Beyond Dolly, other scientific clues hinted that reprogramming was possible. Researchers had demonstrated that transferring the nucleus (the cell's command centre containing DNA) from a somatic cell into an unfertilized egg cell could reset its developmental clock. Similarly, fusing an adult cell directly with an ES cell could sometimes coax the adult cell's nucleus into behaving more like an embryonic one. These experiments showed that something within eggs and ES cells possessed powerful reprogramming capabilities. But what, exactly? It remained largely a black box of unknown molecules. Yamanaka's hypothesis was a curious leap: perhaps this reprogramming wasn't just a vague cellular influence, but could be achieved by introducing a specific, defined set of genes — the very genes that normally maintain the ES cell state.
The experiment
Shinya Yamanaka assembled a team at the Nara Institute to test this theory. The premise of the experiment was simple: for an ES cell to maintain its undifferentiated state, it requires coordinated expression and restriction of certain genes. Pick the right combination of transcription factors — the proteins that latch onto genes to either express or restrict them — and the embryonic stem cell will stay as is, and not differentiate into a specialized cell, like a skin cell to protect your body or a red blood cell to shuttle oxygen around. After differentiation, these “ES maintenance” genes are usually turned off and “locked away”, and other genes that the specialized cell needs to function are then expressed. The question was: if those maintenance genes are “unlocked” and turned on again, will a specialized cell reprogram itself to its ES cell state?
To test this hypothesis, Shinya Yamanaka and his industrious post-doctoral researcher Kazutoshi Takahashi designed a series of clever experiments. They crafted a list of 24 candidate genes that were known or thought to be important in maintaining ES cell state. Their plan was to insert these genes into mouse embryonic fibroblasts (MEFs) — the precursor cells in fetal mice that differentiate into skin, muscle, and bone cells — and check if these induced the stem cell state.
To check whether the transformation was successful, they needed some kind of cellular signal. This required clever engineering: they found a gene that is only active in embryonic stem cells but not necessary for its functioning, named Fbx15, and modified it by combining it with another gene, β-geo, that makes the cell resistant to an antibiotic called G418. This way, if the cell is in an embryonic cell state, it will express the Fbx15 gene, which, now attached with β-geo, will create proteins to neutralize the G418 antibiotic. So now, embryonic stem cells from mice grown with this modified Fbx15 gene could survive very high levels of the antibiotic, but regular, differentiated cells would die even at normal levels. Thus, even a partial activation of Fbx15, indicating a partial move towards ES cell state, would result in the cells being resistant to the antibiotic (at normal levels), and thus distinguishing between ES cells and regular cells. The cells that survive will have gone back in time.
With the signalling mechanism sorted out, they began testing out which of the candidate genes might induce this state. First, they introduced each of the 24 candidate genes into mouse embryonic fibroblasts one by one, through a technique known as retroviral transduction, a method of delivering genes into cells using modified retroviruses1. But when they grew the cultures for each of these, none of the colonies were drug-resistant, suggesting that a single gene may not induce ES cell state.
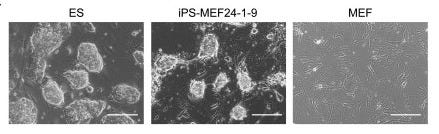
If they didn’t work individually, maybe adding a combination of genes might? Yamanaka and Takahashi jumped to the other extreme: they added all 24 genes together. This time, after 16 days, small specks started to emerge around the dish: colonies. When inspected under a microscope, some of these cells even transformed their morphology, going from the spindle-like structure of MEFs to the round globules of ES cells, with “large nucleoli and scant cytoplasm.” They even started proliferating at the same rate as ES cells. The skin cells seemed to have literally shape-shifted. They called these cells induced pluripotent stem cells, or iPS cells for short, and labelled these specific ones iPS-MEF24.
This stunning result indicated that they were on the right track. Converting a differentiated cell back into a stem cell might indeed be possible. Now, they just needed to find out which of the 24 genes were actually inducing the ES cell state. Working backwards, they began taking out genes one by one; they hypothesized that the ones that stopped colony growth would be critical. Using this elimination method, they were left with 10 genes, and once they combined just these 10 genes — labeled iPS-MEF10 — more cell colonies grew than when they used all 24.
They then repeated the gene pruning process again. Removing genes one by one from the 10, they found that, incredibly, there were just four genes without which colonies wouldn’t grow properly: Oct3/4, Klf4, Sox2, and c-Myc.
Pruning the factors further didn’t work; they found that no combination of two factors would induce colonies, and using three factors sometimes yielded a few , but these were either unstable or looked abnormal — flatter, or rougher and spikier, than true ES cells. Thus, just three factors might initiate the reprogramming journey, but the specific combination of all four was essential for complete, stable transformation into an ES-like state.
So iPS cells using four factors mimicked ES cell morphology and activated the engineered Fbx15 marker gene. But was this resemblance more than skin deep? The critical question remained: were these induced cells truly pluripotent, possessing the defining capabilities of embryonic stem cells?
Answering this required moving beyond appearances and diving into rigorous molecular and functional validation. Real ES cells have characteristic gene expression patterns and the unique ability to differentiate into all cell types; Yamanaka and Takahashi now needed to subject their induced cells to a battery of tests to see if they truly measured up.
RT-PCR
The first test Yamanaka and Takahashi did was an RT-PCR to examine whether the key ES cell “marker” genes were actually being expressed2. The test revealed that in some clones, a majority of the markers that identify ES cells were switched “on”. This can be seen by the indicator bands, with the intensity corresponding to gene expression levels.

Chromatin immunoprecipitation
Next, they performed a test called chromatin immunoprecipitation (ChIP), which is a technique that’s used to study how proteins interact with DNA in living cells. The test helps researchers identify specific DNA regions bound by proteins like transcription factors or modified histones, providing insights into gene regulation and epigenetics3.
To understand what this test reveals requires a review of how DNA is arranged in a cell. DNA isn’t all laid about in a straight line, but wraps around proteins called histones like threads on a spool, creating a complex known as chromatin. When DNA is wrapped around tightly, the genes in that portion are silenced, and when it's packed loosely, genes can be active. Acetylation — adding an acetyl group to histones — loosens its grip, enabling gene activation, and dimethylation — adding two methyl groups to histones — tightens its grip, silencing genes. Thus, cells can use acetylation and dimethylation to control which genes are "on" or "off" without changing DNA.
The analysis revealed a crucial change in the iPS cells at the control regions (promoters) of key pluripotency genes like Oct3/4 and Nanog: histone acetylation had increased, while dimethylation decreased. This meant that the chromatin structure was physically relaxed, making these essential genes more "open" and primed for activation. The epigenetic “locks” were literally loosened.
DNA microarray analysis
A DNA microarray is a tool that also measures gene expression levels, but does so for thousands of genes simultaneously. This allowed the researchers to compare gene expression levels for thousands of genes for ES cells, iPS cells, and MEFs, and assess their similarity. Both a statistical analysis (Pearson correlation4) and the visual patterns — the red-green checkerboard — reveal that iPS cells are clustered closely to ES cells, and both distinct from MEFs. Yet the patterns weren't identical, confirming iPS cells were similar, but not exactly ES cells.
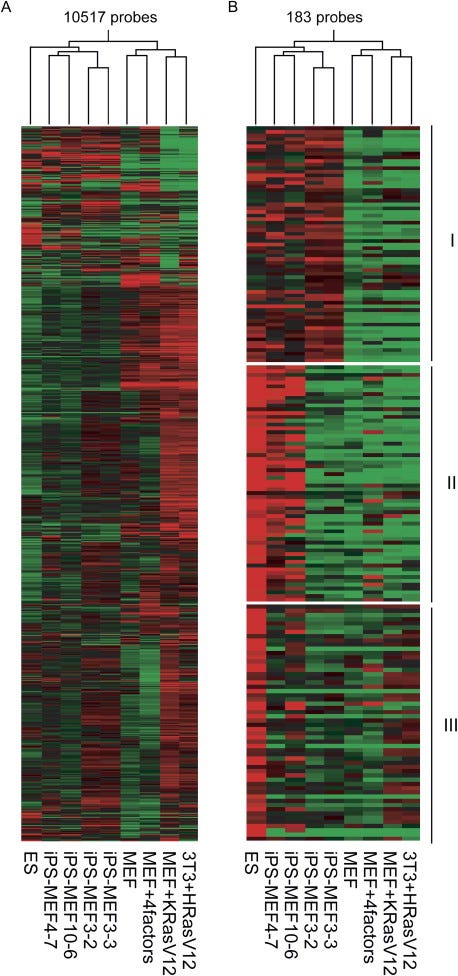
Histological analysis
The next important aspect that Yamanaka and Takahashi wanted to check was whether the iPS cells they created actually do what stem cells do: differentiate into a diverse set of specialized cells. They tested this by injecting the iPS cells into immunodeficient “nude” mice5 — and checking whether they develop into a teratoma, a tumor that includes a disorganized collection of tissues from all three germ layers: the ectoderm, mesoderm, and endoderm. After injecting their cells under the mices’ skin, a histological examination showed that they had indeed differentiated into cell lines starting from all the three germ layers; they found neural tissue (which comes from the ectoderm), cartilage (from the mesoderm), and columnar epithelium (cells that line the digestive tract, which come from the endoderm). Their induced pluripotent stem cells were capable of differentiation.
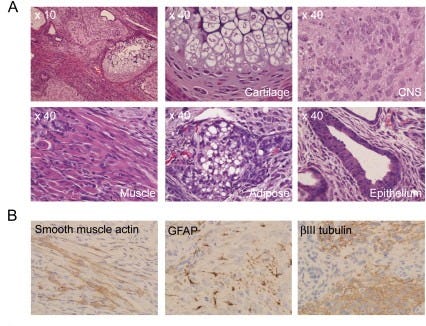
The researchers also decided to do some in vitro tests. All iPS cell lines were cultured in non-coated plastic dishes, where they cannot attach to the bottom, which forces them to form a spherical cluster of cells — in this case, embryoid bodies, which mimic the early stages of embryonic development. When grown in tissue culture dishes, where they can stick to the bottom, the iPS-MEF10 and iPS-MEF4 lines initiated differentiation, but the iPS-MEF3 cells did not. All cell lines confirmed the in vivo experiments.
Using adult skin cells
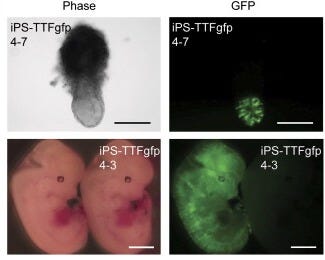
Seemingly satisfied with the results so far, the pair of researchers now went to change something that’s been constant from the start. Rather than test on mouse embryonic fibroblasts — cells derived from early embryos that retain some developmental flexibility — they decided to ask whether you could induce pluripotency from a fully specialized adult cell. In this case, they used tail-tip fibroblasts, the skin and tissue cells from a mouse’s tail. They injected the same four factors into four 7-week-old male mice, as well as one 12-week-old female mouse which continuously expressed green fluorescent protein. From the male mice, they established a line of iPS cells, which they called iPS-TTF4, and from the female mouse, they established 6 iPS cell lines (called iPS-TTFgfp4).
Under a microscope, these cell lines were indistinguishable from ES cells. Similar validation tests were performed on these adult-derived iPS cells: RT-PCR confirmed the expression of key ES cell markers, while teratoma assays showed their ability to differentiate into all three germ layers, mirroring the results seen with embryonic-derived iPS cells. They also injected the gpf version into mouse blastocysts, some of which developed in glowing mice embryos.
More tests
Incredibly, both mouse embryonic fibroblasts and adult mouse skin cells are undeniably capable of turning into pluripotent stem cells; it’s possible to reverse biological time. This itself would be an incredible discovery, but the researchers didn’t stop there. They wanted to find out in more detail what the differences were and whether they were crucial or not. They wanted to paint a portrait of this new cell.
Yamanaka and Takahashi performed more tests:
A real-time PCR test revealed that the introduced factor genes were highly active, yet total protein levels mirrored those in ES cells, suggesting sophisticated internal controls managing the over-expression.
A Southern blot analysis showed that each iPS clone had a unique pattern of where the four factors were inserted into the genome. The different insertion locations confirmed that the iPS cells were independently generated and not duplicates of one another.
Karyotyping analysis6 showed that most iPS clones had a normal set of 40 chromosomes, but some clones had abnormalities, such as missing or extra chromosomes7.
These characteristics — gene expression differences and unique genetic fingerprints — ruled out the possibility that these iPS cells were accidentally contaminated with preexisting ES cells8.
These series of tests also solidified the iPS cell's identity as a novel, robust entity. But another fundamental question lingered: how did just four factors trigger such a profound cellular reset?
The architects of induced pluripotency
The earlier discovery of loosened epigenetic "locks" held a clue. One of the new additions in the “reprogramming toolkit”, c-Myc, does not directly target genes that are required in reprogramming but plays the role of a “genetic crowbar”, prying DNA from their histones and opening up the genome to make it easier for Oct3/4 and Sox2 to reach their targets. Its other role is to activate genes that drive cell growth and division, which help cells multiply during reprogramming.
The final key protein, Klf4, balances two opposing roles: activating stem cell genes and managing cell survival versus growth. Klf4 blocks p53, a protein that normally suppresses stem cell genes. Since iPS cells have lower p53 levels than regular cells, this allows these other stem cell genes to stay active, maintaining the cells’ reprogrammed state. Klf4 also slows cell division by activating p21CIP1, a protein that halts cell growth. c-Myc counteracts this by blocking p21CIP1, allowing cells to keep dividing during reprogramming. This balance between Klf4’s “brake” and c-Myc’s “gas pedal” is crucial: too much Klf4 could stall growth, while too much c-Myc might kill cells. Together, they help reprogram cells efficiently without derailing the process.
The reprogramming lottery
Despite the promising results, a key puzzle remained: why did only a fraction of treated cells actually become iPS cells? Yamanaka and Takahashi wondered whether only rare, pre-existing stem cells within skin cells (~0.067%) were being converted. Re-testing using stem-cell-rich bone marrow, however, yielded similarly low efficiency, suggesting the bottleneck wasn't the starting cell type, but the reprogramming process itself.
So, what, then, makes it such a rare event? They considered a few possibilities. Transformation might require hitting a precise "Goldilocks zone" for the four factors' activity. While the cells do have sophisticated internal controls to tightly manage their protein levels, there are limits to those controls. Additionally, reprogramming could rely partly on chance. Perhaps spontaneous genetic mutations during culture play a role, or maybe it’s the retroviruses themselves acting as genomic wildcards. Inserting their DNA cargo haphazardly across the genome (~20 times per cell!), they could accidentally disrupt important genes or activate harmful ones. This reliance on precise internal conditions and unpredictable genetic events likely explains the low efficiency, highlighting the need for more controlled methods to improve it.
Another possibility was that nature's reprogramming recipe in egg cells might be more complex than just four factors. Incredibly, two factors which are crucial in iPS cells — c-Myc and Klf4 — are not important in egg cells; in fact, c-Myc isn’t even present at all. Egg cells do, however, contain cousins of these factors — L-myc and Klf17 or Klf7 — suggesting that these might fill in for the others, like backup actors taking over (or were those the backup actors?). Egg cells are totipotent — capable of creating the entire organism as well as the organs required for its development, like the placenta — so maybe these other factors are required to potentially reprogram a cell into a totipotent cell.
More pressingly, they also examined whether the introduced factors would switch off safely after differentiation. They found that in iPS cells, epigenetic silencing could be leaky, particularly for the cancer-linked c-Myc. It underscored that harnessing this power for human treatments would require further refinement, perhaps finding alternatives to risky factors. Nevertheless, this discovery fundamentally altered the landscape, paving the way for patient-specific cells to fight disease and offering a tangible path towards the dream of personalized medicine.
A revolution in a dish
Today, these four factors that induce pluripotency — Oct3/4, Klf4, Sox2, and c-Myc — are known as Yamanaka factors. Just a year after publishing this paper on mice, in 2007, Yamanaka and his team successfully reprogrammed human skin cells into iPS cells using the same four factors. The breakthrough earned Shinya Yamanaka the Nobel Prize in Physiology or Medicine in 2012, just 6 years after the original discovery was published.
In the nearly two decades since the discovery, iPS cells have been crucial in many key advancements and applications: disease modeling, gene and cellular therapy, drug discovery and testing, and personalized and regenerative medicine. For example, in 2011, researchers from Kyoto University created iPSCs from patients with Alzheimer's disease and differentiated them into neurons, allowing them to study the disease process in a dish. Ongoing iPSC research and development is also focused on blood transfusion applications like creating red blood cells, platelets, and addressing alloimmunization risks.
Even Yamanaka, driven from medicine into research by his father's death from an untreatable disease, couldn't have foreseen the seismic impact of his work. His goal was to understand disease, hoping this might pave the way for future cures. The induced pluripotent stem cell delivered that and infinitely more — sidestepping embryo debates, creating avenues for personalized cures from a patient's own cells, and revolutionizing how we study disease and test drugs9.
This essay sought to illuminate not just the what, but the how. Science, particularly at the cutting-edge, is a demanding, iterative process. Discoveries aren’t so much stumbled upon as they are wrought. The headline “Scientists Bypass Need for Embryo” couldn't convey the years spent asking the question: “is this real?” The slew of tests wasn't just procedural; it was an essential act of validation, of creative problem-solving, and of intellectual honesty — the qualities required to answer that question with some degree of certainty. It's a story of immense effort, often invisible to those outside the lab. Yamanaka's personal motivation doubtlessly fueled the dedication required for this scientific marathon, ultimately creating a technology that heralded a future of medicine his father could only have dreamed of.
You might also like:
I should have loved biology too
About a year ago, I came across James Somers’ blog post, I should have loved biology. I began reading it and every sentence struck a chord: “I should have loved biology but found it a lifeless recitation of names”; “In textbooks, astonishing facts were presented without astonishment”; “In biology class, biology wasn’t presented as a quest for the secret…
Essays #1: Dancing in E. Coli
There is a unique joy that comes from learning a topic in more detail. You’ll have a rough idea of what something is, and then a revelation — a paragraph or chapter or video or conversation — will make you realize that there’s so much more to it than meets the eye. It feels, almost, like a gentler, prolonged ‘eureka’ moment. Your mind perks up, excited …
The virus's harmful genes are removed and replaced with the gene(s) of interest. These modified viruses then 'infect' the target cells, inserting the desired genetic material directly into the host cell's DNA. The cell then treats this inserted DNA like its own, potentially expressing the new genes.
RT-PCR is actually two techniques put together – reverse transcription (RT) and polymerase chain reaction (PCR). Reverse transcription is a way to convert RNA extracted from the cell into complementary DNA (cDNA) using enzymes called reverse transcriptase, and PCR is a technique to amplify certain genes of interest from the cDNA. The end result is that you can analyze the expression levels of specific genes. (By design, the primer they used only amplified endogenous genes and not transgenes)
How it works: Proteins and DNA are chemically "glued" together (usually with formaldehyde) to preserve their natural interactions; then, the DNA-protein complexes are broken into small pieces (~300–1,000 base pairs) using sound waves (sonication) or enzymes; then, an antibody specific to the protein of interest (a transcription factor or modified histone) is used to "fish out" the protein and its attached DNA from the mixture; and finally, The protein is removed, and the bound DNA is isolated for analysis (PCR, microarrays, sequencing).
A Pearson correlation analysis is a statistical method used to measure the strength and direction of the linear relationship between two continuous variables. In simple terms, it helps determine how closely two variables are related to each other.
The mice have a genetic mutation that doesn’t allow them to develop a thymus, thus making them immunodeficient, which incidentally doesn’t let them grow body hair
Karyotyping is a method to check the number and structure of chromosomes in cells.
They also found that iPS cells could not maintain their undifferentiated state without feeder cells, even when leukemia inhibitory factor (LIF), which supports stem cell growth, was present.
They even grew clones of the iPS clones – subclones – and found that those too differentiated into all three germ layers, again confirming that they were true pluripotent stem cells
The scientists that found hepatitis C in 1989 got their Nobel Prize for their discovery in 2020, so he incredibly got his before the scientists and the discovery that motivated him to become a scientist in the first place.







This was excellent, thanks for beautifully summarizing one of the most important stories of biology.